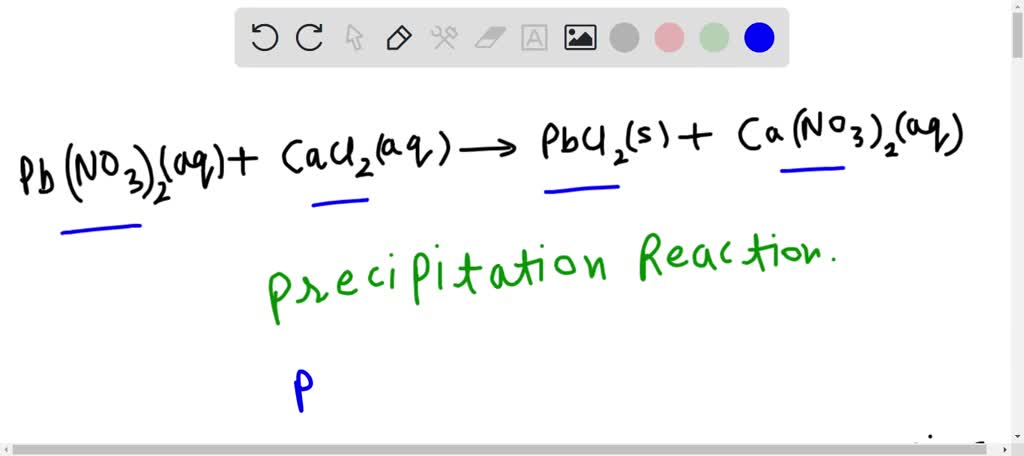
organic chemistry - What is role of copper powder, calcium chloride and cuprous chloride in the SN1 reaction of hydrochloric acid with propargylic alcohol? - Chemistry Stack Exchange

Pyrohydrolysis of CaCl2 Waste for the Recovery of HCl Acid upon the Synergistic Effects from MgCl2 and Silica | ACS Sustainable Chemistry & Engineering

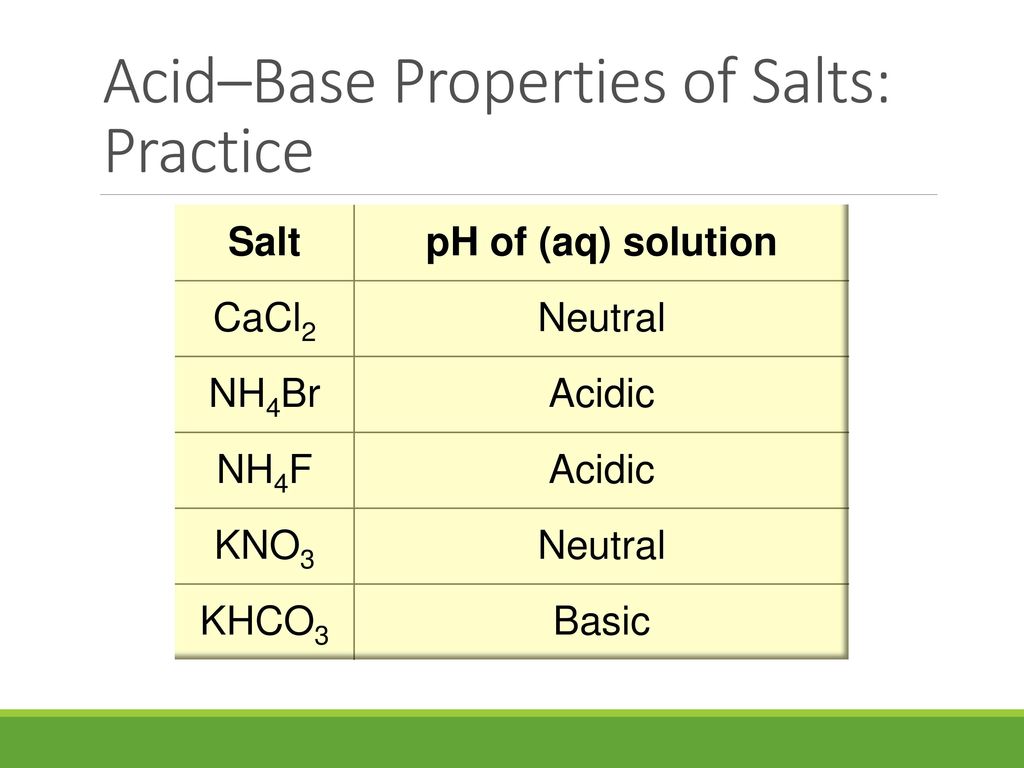
Mean values of pH in CaCl2, saturation per base and potential acidity... | Download Scientific Diagram

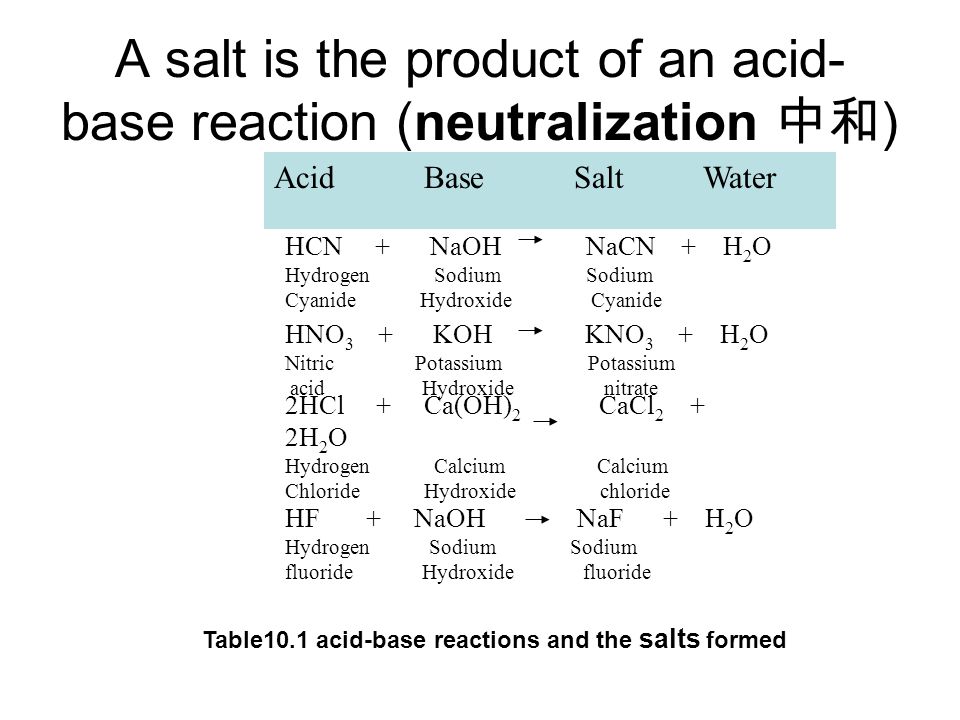
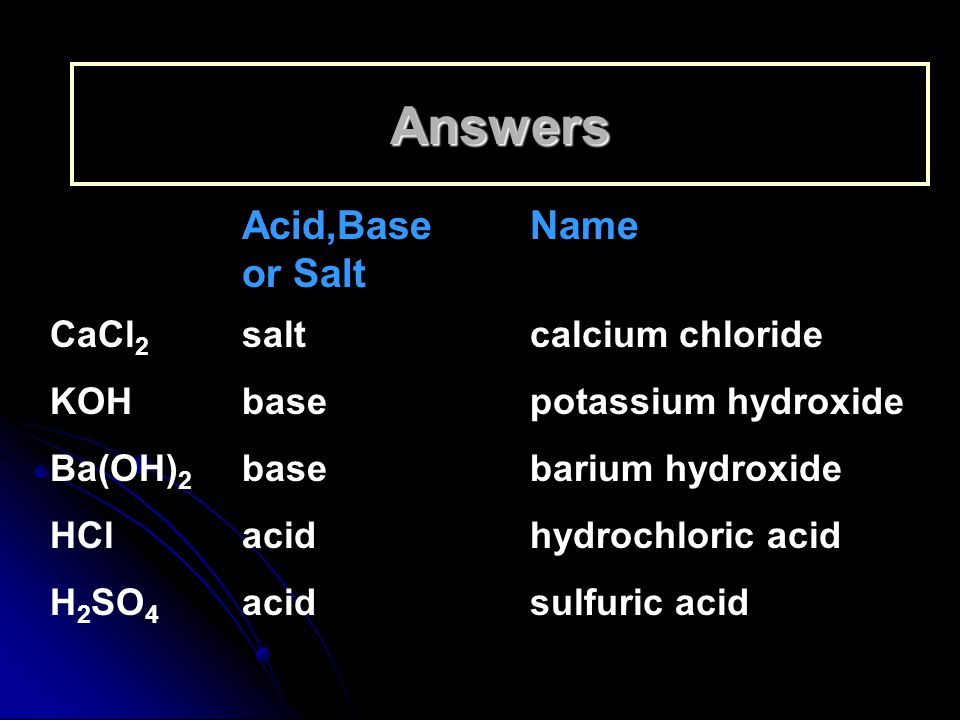
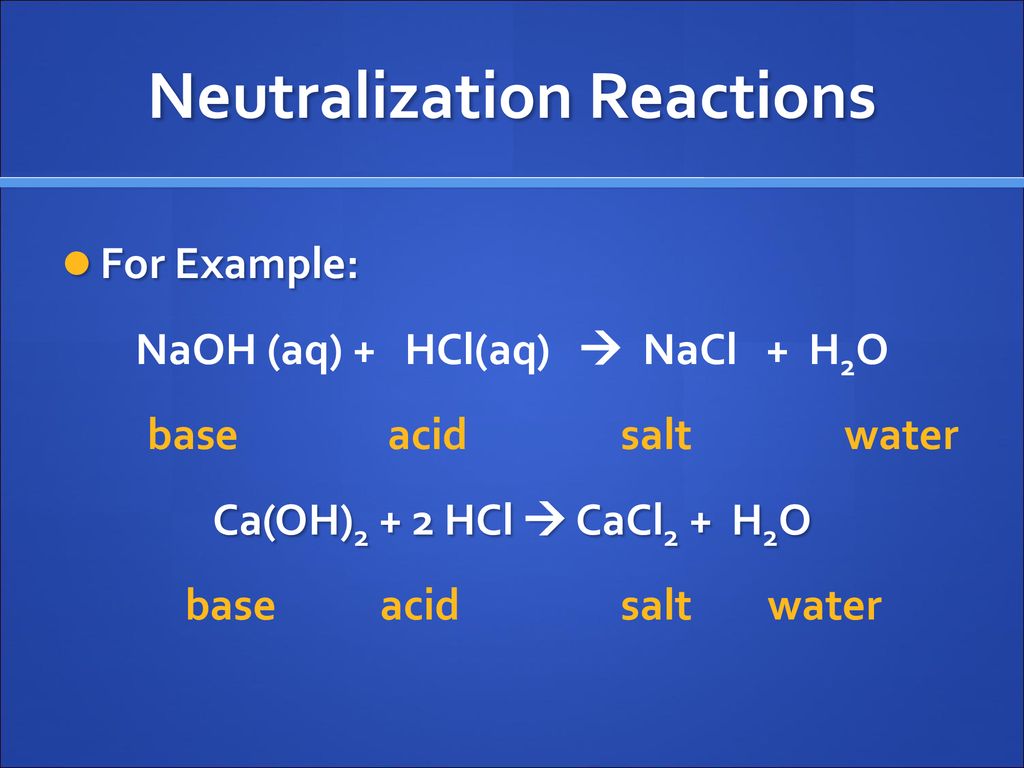
Name the acids and bases from which the following salts may be obtained. (i) Potassium sulphate (ii) Calcium chloride

Question Video: Calculating the Mass of Calcium Chloride That Contains a Given Mass of Chlorine | Nagwa

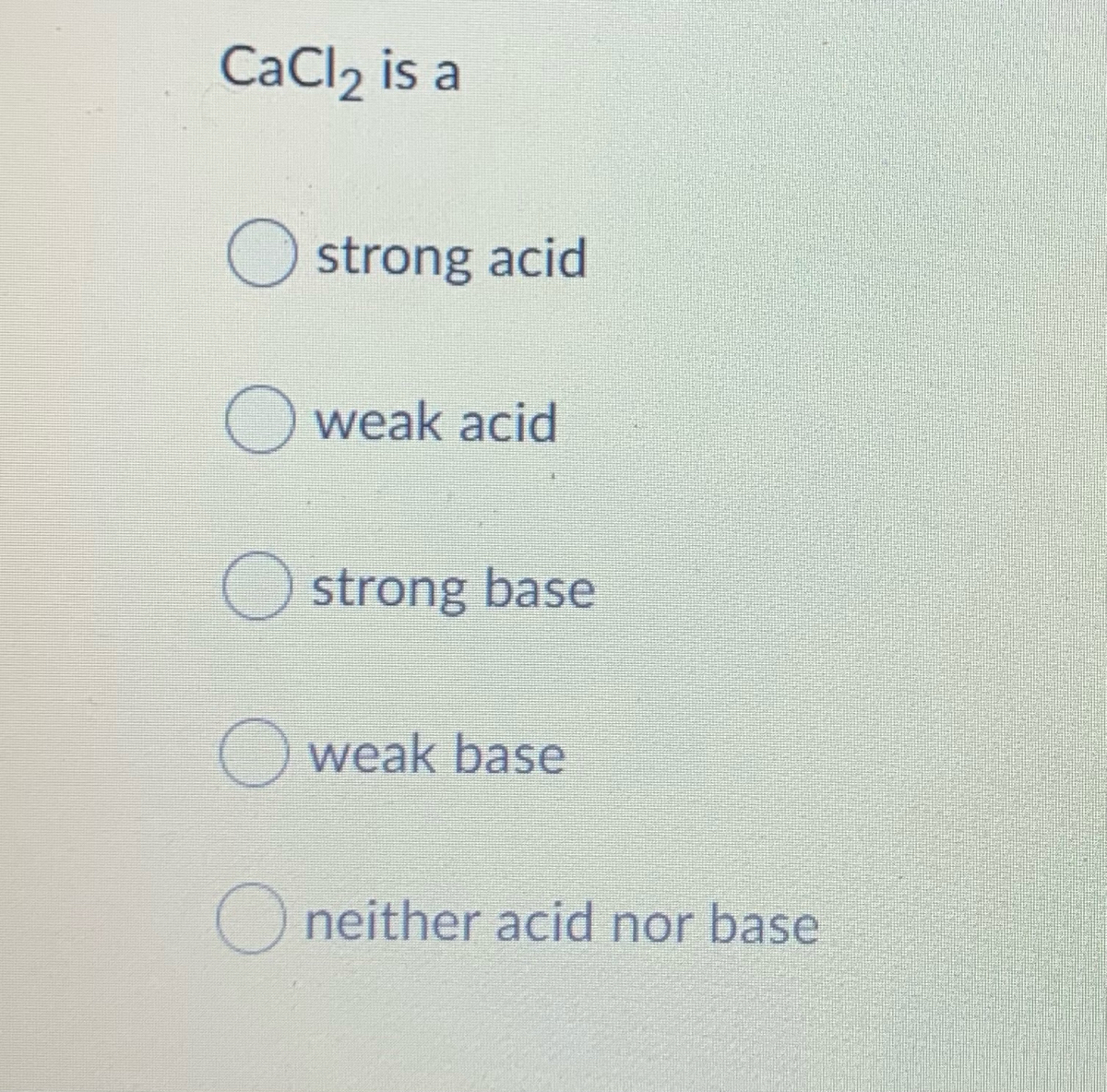
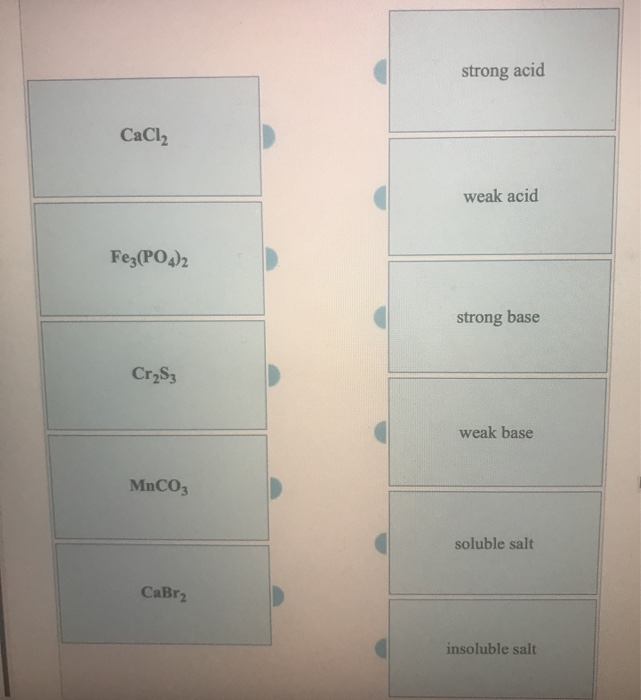
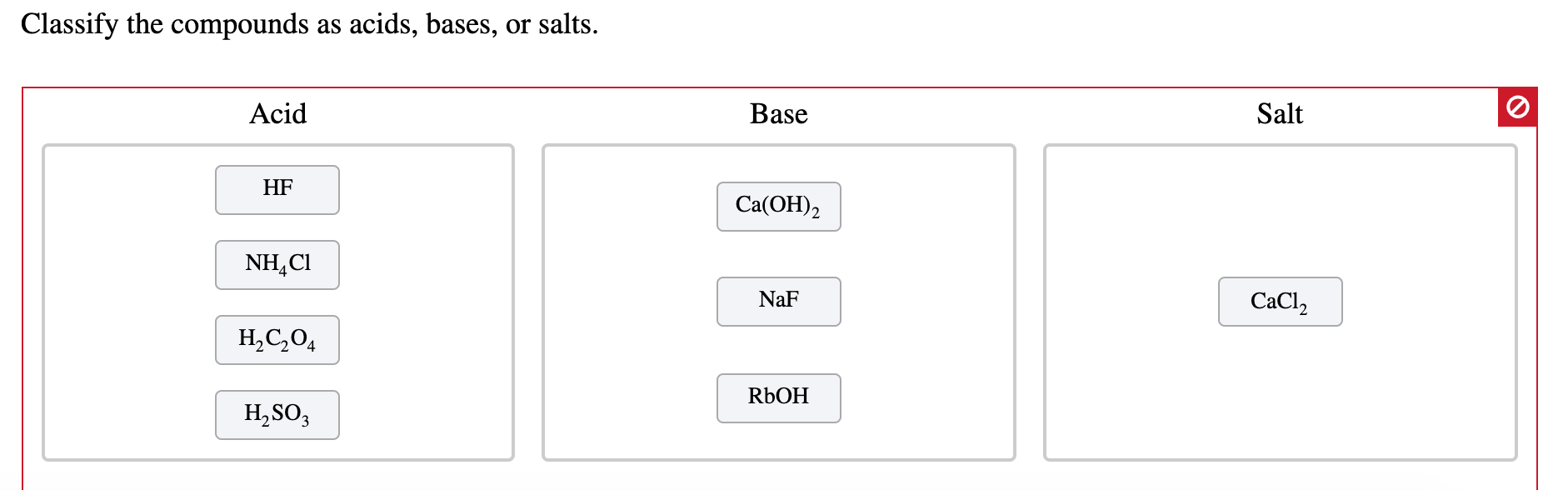
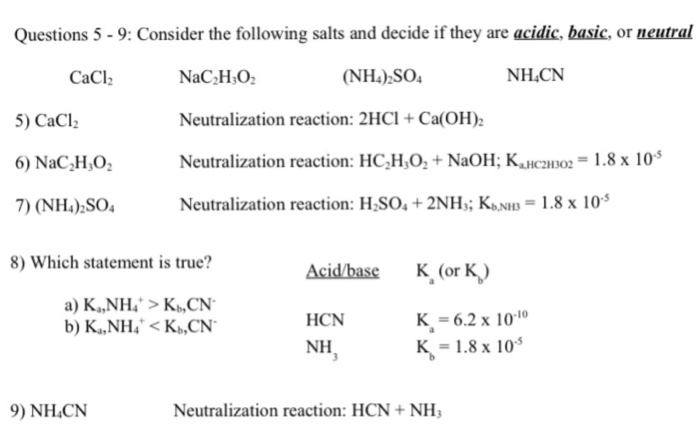
SOLVED: Classify the compounds as acids, bases, or salts. Which are Acids? Which are Bases? Which are Salts? Answer Bank LiCl KOH HBr Ba(OH)2 CaCl2 H2SO3 NH4NO3 H2C2O4